Synthroid has been committed to treating hypothyroidism for over six decades.8
- It’s America’s #1 prescribed branded oral medication7*
- Millions of patients manage their hypothyroidism with Synthroid9†
*2015-2024.
†2024.

Synthroid has been committed to treating hypothyroidism for over six decades.8
*2015-2024.
†2024.

Synthroid has been committed to treating hypothyroidism for over six decades.8
*2015-2024.
†2024.
Synthroid has been committed to treating hypothyroidism for over six decades.8
*2015-2024.
†2024.
In August 2002, the FDA released the Current Good Manufacturing Practices (CGMP) initiative to ensure the quality of drug products. The initiative contains minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packing of a drug product. These regulations make sure that a product is safe for use, and that it has the ingredients and strength it claims to have.5,6
AbbVie is committed to adhering to the CGMP and believes in the integrity of its manufacturing process. AbbVie is dedicated to ensuring that all Synthroid products are manufactured at the highest standards.
Note that FDA regulations require that all pharmaceutical manufacturers like AbbVie abide by CGMP.
*Mistaken generic users defined as those who reported taking Synthroid but were not taking a pill with the word “SYNTHROID” embossed on it. From a 2021 national online survey of 1908 adults diagnosed with hypothyroidism and currently taking LT4 products.10
That’s because substitutions can be made at the pharmacy if the prescription is not properly protected with the Dispense and Written (DAW) state-specific language.
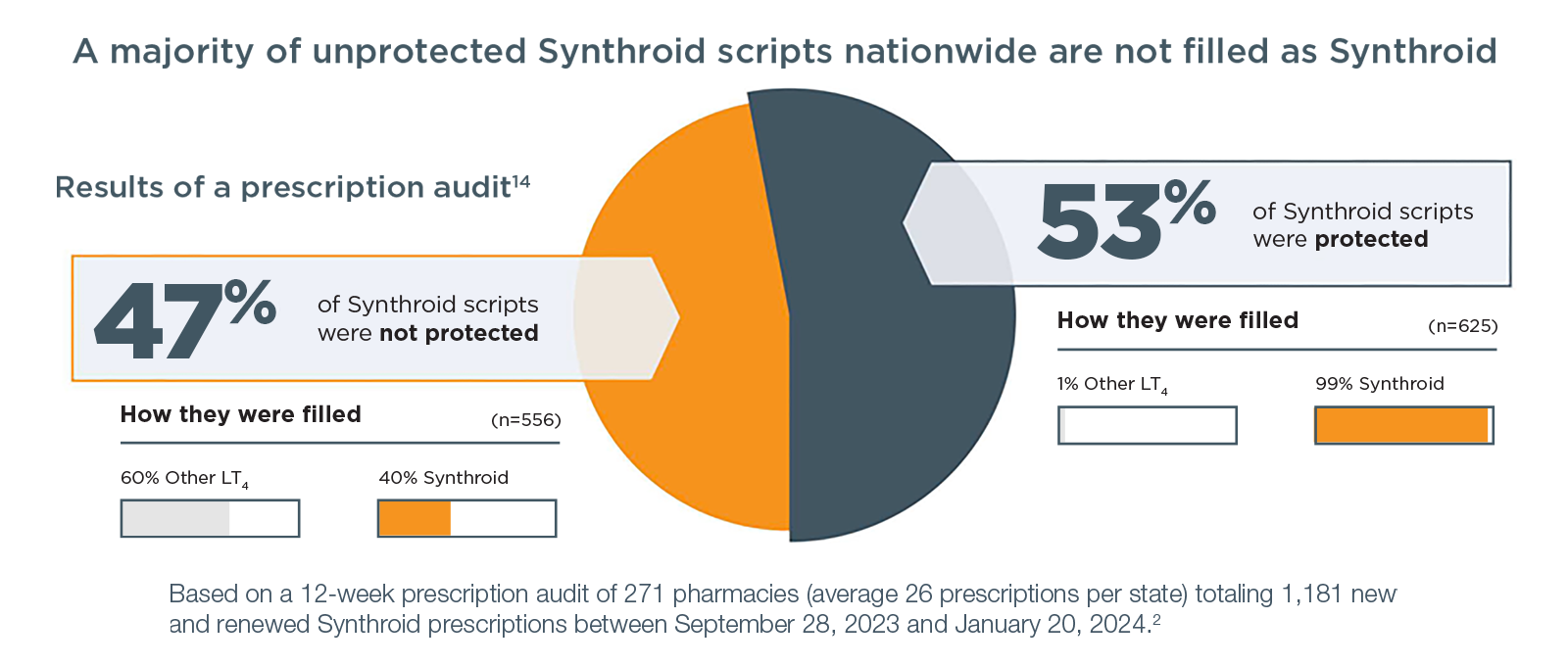
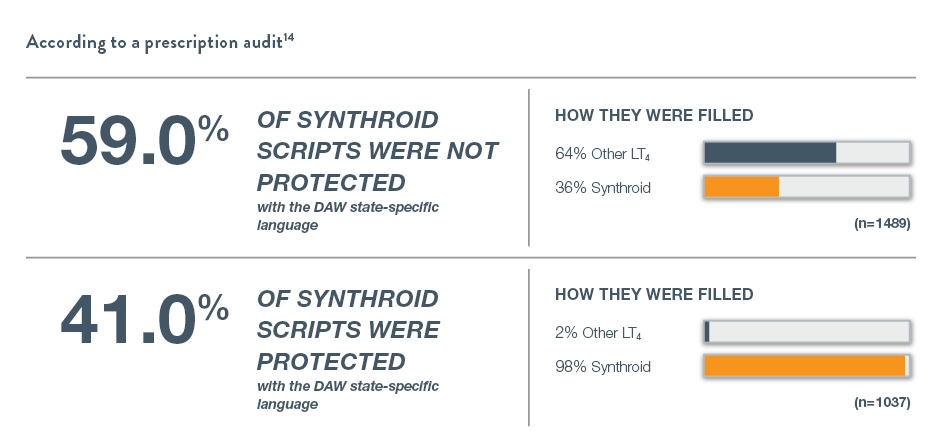
The FDA has determined that certain levothyroxine products are interchangeable. The FDA has determined that drugs that are classified as therapeutically equivalent can be substituted with the full expectation that the substituted product will produce the same clinical effect and safety profile as the reference product.15
DAW codes are codes a pharmacy uses when filling your patient’s prescription. DAW-1 indicates that substitution is not allowed, based on the prescriber’s preference, and ensures your patient will receive the treatment you prescribe. It also helps your patient pay the lowest possible price for the product you write if it is billed through insurance.
When prescribing Synthroid, protecting your script can ensure your patients receive Synthroid every time they refill their prescription.
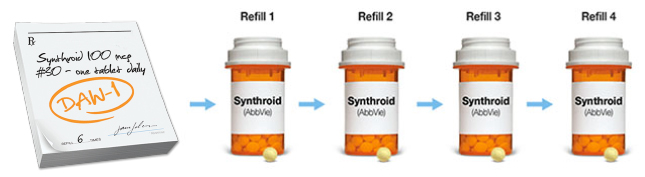
Tablets shown not actual size and may not represent exact color.

Hypothyroidism
SYNTHROID® (levothyroxine sodium) tablets for oral use is an L-thyroxine (T4) indicated in adult and pediatric patients, including neonates, as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
Pituitary Thyrotropin (Thyroid Stimulating Hormone, TSH) Suppression
SYNTHROID is indicated in adult and pediatric patients, including neonates, as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer.
SYNTHROID is not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients, as there are no clinical benefits and overtreatment with SYNTHROID may induce hyperthyroidism.
SYNTHROID is not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis.
Thyroid hormones, including SYNTHROID, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
US-SYNT-220356